Conservation of Matter
Grade 8 Science Worksheets
Molecules and Matter
The Universe is made of Matter. All matter occupies space. Mass is the amount of matter in an object. It is the sum of all the Molecules which make up that matter. Molecules are packed differently in different matter.
Schedule a Free session to clear worksheet doubts
No credit card required, no obligation to purchase.
Just schedule a FREE Sessions to meet a tutor and get help on any topic you want!
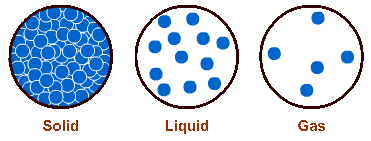
Solids, for example, are packed densely with molecules – these molecules do not move from one part of the matter to another. They have a definite shape and they occupy a definite space.
In liquids, molecules move about a bit more easily, but yet not far apart from one another. Liquids, therefore, have an indefinite shape but they still occupy a definite space.
Gas molecules move far apart from one another, they collide and repel one another and fill up all the space available to them, such as a jar containing ammonia gas. They, therefore, have no shape at all, and consequently they occupy indefinite space.
Volume and Density
The amount of space occupied by an object is called its Volume. The amount of mass fitted into a particular volume determines the Density of that matter.
In other words, Density = Mass/Volume.
Solids have higher density than liquids – their molecules are closer together – and liquids have higher density than gases. Therefore, for the same amount of mass, solids tend to occupy lesser space than liquids and liquids occupy lesser space than gases.
Learn more about Conservation of Matter and other important topics with 8th Grade Science Tutoring at eTutorWorld. Our expert science tutors break down the topics through interactive one-to-one sessions. We also offer the advantage of customized lesson plans, flexible schedules and convenience of learning from home.
Personalized Online Tutoring from eTutorWorld
eTutorWorld offers affordable one-on-one live tutoring over the web for Grades K-12, Test Prep help for Standardized tests like SCAT, CogAT, MAP, SSAT, SAT, ACT, ISEE and AP. You may schedule online tutoring lessons at your personal scheduled times, all with a Money-Back Guarantee. The first one-on-one online tutoring lesson is always FREE, no purchase obligation, no credit card required.
For answers/solutions to any question or to learn concepts, take a FREE TRIAL Session.
No credit card required, no obligation to purchase.
Just schedule a FREE Sessions to meet a tutor and get help on any topic you want!
The Conservation of Matter
The Law of Conservation of Matter states that matter can neither be created nor destroyed. Matter can change form through physical or chemical changes but in both cases, the matter is conserved.
Take the example of a physical change – water freezes to form ice or ice melts to form water. In both these changes, the number of molecules of water remains exactly the same before and after the physical change. In other words, the matter is neither created nor destroyed.
Now let’s look at a chemical change – hydrogen and oxygen atoms react chemically to form water. It is represented by a balanced chemical equation as below:
2H2 (g) + O2 (g) → 2H2O (l)
The equation shows that 4 atoms of hydrogen or 2 molecules of hydrogen gas react with 2 atoms of oxygen or one molecule of oxygen gas, resulting in 2 molecules of a liquid named water, each molecule containing 2 atoms of hydrogen and one atom of oxygen. In other words, the total number of hydrogen and oxygen atoms (4 and 2 respectively) remains exactly the same before and after the chemical reaction.
The same number of atoms of hydrogen and oxygen before a chemical reaction gets arranged differently to form a new substance of matter – water. Yet, the total mass remains the same, once again proving the law of conservation of matter.
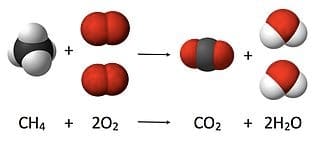
A balanced chemical equation for the combustion of methane with the same number of atoms of elements before and after the reaction, demonstrating the law of conservation of matter.
Check Point
- The amount of matter in an object determines its _____.
- The amount of space occupied by an object is known as its ______.
- The amount of mass fitted into a particular volume is called its ______.
- For the same amount of mass, the matter having the least density would be –
- Liquid
- Gas
- Solid
- ______ can neither be created nor destroyed as a result of a physical or chemical change.
Answer Key
- Mass
- Volume
- Density
- b) Gas
- Matter
Schedule a Free session to clear worksheet doubts
No credit card required, no obligation to purchase.
Just schedule a FREE Sessions to meet a tutor and get help on any topic you want!
Learn more about Scientific Method and other important topics with 7th Grade Science Tutoring at eTutorWorld. Our expert science tutors break down the topics through interactive one-to-one sessions. We also offer the advantage of customized lesson plans, flexible schedules and convenience of learning from home.
Pricing for Online Tutoring
| Tutoring Package | Validity | Grade (1-12), College |
|---|---|---|
| 5 sessions | 1 Month | $124 |
| 1 session | 1 Month | $25 |
| 10 sessions | 3 months | $239 |
| 15 sessions | 3 months | $354 |
| 20 sessions | 4 months | $449 |
| 50 sessions | 6 months | $1049 |
| 100 sessions | 12 months | $2049 |
8th Grade Free Worksheets
- The Universe
- Heredity
- Evolutionary Theory
- Structure of the atom
- Ethical Practices
- Unveiling the mystery behind the physical universe
- Components of the universe
- Celestial phenomena
- The tilt of Earth’s axis
- The causes of high and low tides
- Earth Systems
- Rocks and Fossils
- Weather and Climate
- Basics of chemical reactions
- Types of Chemical reactions – Endothermic, exothermic, oxidation, reduction reactions
- Catalysts and enzymes
- Compounds and mixtures
- Acids, Bases and pH Indicators
Images Credit:
https://pngimage.net/wp-content/uploads/2018/06/matter-png-5.png
https://upload.wikimedia.org/wikipedia/commons/7/7c/Combustion_reaction_of_methane.jpg


